A PSC Partners-Sponsored Study to Accelerate Drug Development for PSC
What Do I Need To Know About WIND-PSC As A Patient Or Caregiver?
Please read our WIND-PSC overview document for patients and caregivers to learn more.
What Is The WIND-PSC Initiative?
In 2022, PSC Partners unveiled the WIND-PSC initiative, a PSC Partners-led and sponsored initiative. WIND-PSC was inspired by our community's call to identify new treatment options for PSC and address the unmet medical needs of PSC patients. WIND-PSC has been developed to generate key data to remove barriers and accelerate the development of new treatments.
Through the 2020 Our Voices survey, PSC Partners asked patients to choose the most important outcome, beyond a cure, from developing new medications. More than 70% of PSC patients said that the most important area of focus is the development of treatments to slow the progression of their PSC. With multi-stakeholder support and participation in the WIND-PSC initiative, PSC Partners aims to accelerate drug development for PSC, taking vital steps to solve this core, patient-identified need. Currently, there are no regulatory-approved therapies for PSC, and developing new treatments remains challenging.
The WIND-PSC study has been under development since 2022 and was launched in 2024. As of March 2025, the WIND-PSC Study, a Global Prospective Cohort to Support the Development of New Treatments for Primary Sclerosing Cholangitis, is active at study sites in America, Canada, and Germany. As new study sites are added to WIND-PSC, they will be listed on the ClinicalTrials.gov study record: NCT06297993.
WIND-PSC is creating a database and biorepository to serve as a central location for the broad PSC research community to utilize PSC clinical data and patient-reported symptom experiences from a large prospective cohort of people with PSC.
WIND-PSC Goals
Primary Objective: Develop an appropriate real-world data comparator cohort to support the design, execution, and serve as an external control for interventional clinical trials in PSC.
Secondary Objectives:
- Develop a large clinical and biomarker data set to identify individual and/or composite surrogate endpoints likely to predict clinical benefit for use in the design of interventional studies in PSC.
- Evaluate patient-reported symptoms, quality of life (QoL), and other direct patient experiences with standardized tools to determine changes over time, the association with clinical events, biomarkers, and disease progression, and confirm the psychometric properties of the PRO measures.
Why Did PSC Partners Develop the WIND-PSC Initiative?
PSC Partners is expanding its approach to research funding. In addition to the annual call for proposals and grant funding, the organization has launched the International Collaborative Research Network (ICRNetwork) and developed a strategic research plan (SRP). In developing the SRP, the PSC Partners Board of Directors and staff identified the WIND-PSC initiative as a clear priority for finding treatments for PSC. Together with the support of both PSC Partners and PSC Partners Canada, the launch of the WIND-PSC initiative is community-funded.
Why was this project prioritized?
- PSC patients’ lived experience, without effective treatment, is important evidence of the natural course of PSC progression.
- At some future date, when a treatment for PSC is approved, there may be a need for a post-marketing surveillance trial. Such a trial will require a control arm of PSC patients receiving a placebo, not the new treatment. Through the WIND-PSC initiative, there is a possibility for patients from this WIND-PSC cohort to replace part or all of the control arm. This is called an external control arm.
- Why now? A number of therapeutics are in clinical trials for PSC, making now the best time to begin collecting data to serve as an external control arm.
Which Problems Will PSC Partners Aim To Solve For The PSC Community?
PROBLEM: Existing natural history data are segmented and difficult to share.
SOLUTION: PSC Partners will work tirelessly to build collaborations and bring data together.
- Our PSC patient data is precious; we simply can’t afford to let it sit on the sidelines at individual institutions. Each data point represents a patient’s life and experience, and we hope they each will be used to the greatest extent possible to pursue new treatments for PSC.
- As a neutral third party, PSC Partners can work with researchers and their institutions to integrate data.
- In particular, as sponsors and stewards of the WIND-PSC data, PSC Partners and the multi-stakeholder steering committee enable participating sites to share data in a centralized PSC Partners database to be used for future research.
- PSC Partners is working with Arbor Research to serve as the data coordinating center and will organize a panel of experts and PSC community members to review and approve data requests. This process will ensure that data is deidentified and shared safely and anonymously.
PROBLEM: Resources are limited, which impedes our ability to recruit patients and collect data.
SOLUTION: PSC Partners, with the support of the PSC community, will invest funding to support research sites and ensure rigorous data collection.
- Across the world, dozens of research institutions have already begun collecting PSC natural history data. Excitingly, these have led to multiple publications and increased our understanding of PSC.
- At the same time, researchers at these sites do not have unlimited funding to support experienced personnel to extract and manage the data.
- Through the initial commitment of $4 million U.S. dollars in PSC community funding, PSC Partners supports our academic partners in bolstering their ability to collect, extract, and share the highest-quality data into the PSC Partners database.
PROBLEM: Researchers share core values, but creating a unified effort has been a challenge.
SOLUTION: PSC Partners will serve as an advocate and representative for PSC patients as a neutral third party, uniquely motivated to create a unified effort.
- With so many important areas of ongoing research, dozens of researchers are studying the natural history of PSC. Some natural history registries focus on understanding untreated PSC, some on the relationship between PSC and IBD, and others aim to understand the effects of PSC on quality of life.
- With so much going on at individual sites, differing legal environments, and limited resources, creating a shared database has been challenging.
- PSC Partners, as a neutral third party, is uniquely positioned to lead this effort with their sole focus on PSC.
Who Is Involved In WIND-PSC? Where Can I Join?
WIND-PSC is the inaugural project of the PSC Partners International Collaborative Research Network (ICRNetwork). Its success relies on support from patients, caregivers, clinicians, and researchers.
In order to support drug development, PSC Partners is in the process of establishing formal partnerships with up to 20 academic researchers and institutions. Please see ClinicalTrials.gov study listing here for the sites.
It is important to know that the goals of WIND-PSC can be accomplished through enrolling a few thousand dedicated PSC patients who are willing and able to follow up regularly at dedicated research centers. It’s possible, however, that given the limited budget, geographical considerations, and the number of patients and research centers needed to accomplish these goals, not all interested PSC patients will be able to join this initial cohort.
At PSC Partners, we value every PSC patient's voice and experience. We are so inspired by the dedication of our patient community and the interest that many have already shown in joining this effort. We encourage all people with PSC, regardless of location, to join the PSC Partners Patient Registry, where their participation will contribute priceless input and support to the WIND-PSC project and to other PSC research studies. Through the Registry, PSC Partners has the ability to connect PSC patients to ongoing natural history registries, research studies, and clinical trials for PSC.
Ultimately, PSC Partners hopes that patient participation in the PSC Partners Patient Registry, combined with the current focus of launching this mighty WIND-PSC cohort, will provide a strong foundation for future efforts to expand and enroll all interested patients into observational cohorts.
When Will The WIND-PSC Cohort Begin Recruiting? When Will It End?
PSC Partners is pursuing an aggressive timeline to accomplish the goals of WIND-PSC. While the dates will depend on various factors coming together as the study launches at all sites and patients enroll, here’s our best estimate:
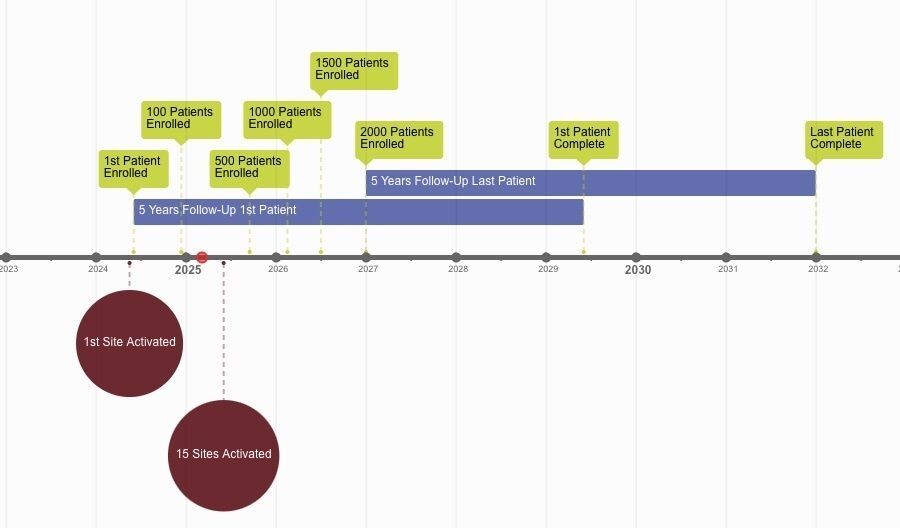
March 2022: Begin drafting the WIND-PSC protocol with expert researchers. Begin pursuing data sharing agreements
2024: Begin contracting with individual sites and begin enrolling PSC patients at participating research centers
2025: Complete contracting with up to 20 sites
2026: Complete enrollment of 2,000 patients into WIND-PSC
2028: Begin interim analyses and begin using data to support drug development
2031: Complete 5-year follow-up and pursue additional directions for WIND-PSC
2031 +: Expand and leverage WIND-PSC to address additional needs of the PSC community
What Can I Do To Support WIND-PSC?
Speak Up
Are you excited to join the Registry and hear more about the WIND-PSC cohort? We’d love to hear from you on social media: tag us and use the hashtag #WIND-PSC. Please also share information about WIND-PSC and the Patient Registry, including a link to this webpage, with your care team.




