PSC Partners Research Grants Program Funding Milestones
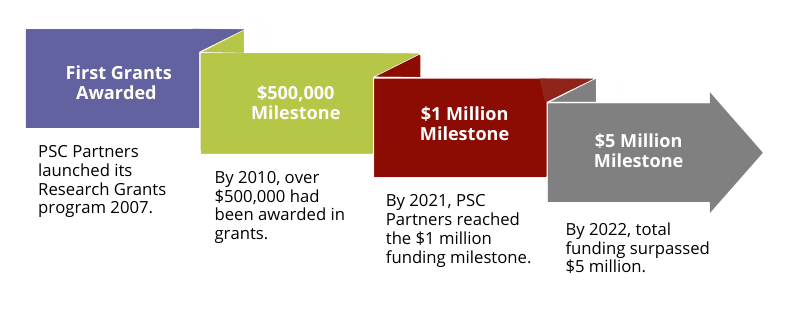
- In 2007, PSC Partners officially announced the launch of the Research Grants program, which aims to fund seed grants for novel research projects to improve our understanding of disease progression and find a cure for PSC.
- In 2009, PSC Partners awarded three grants of $40,000 each to researchers from the Mayo Clinic, Rochester, the University of California, Davis, and the Academic Medical Center in the Netherlands.
- In 2016, PSC Partners Canada joined hands with us to fully fund their first research grant.
- In 2018, PSC Partners and PSC Partners Canada launched the Young Investigator Award category to fund up to $80,000 for promising researchers in the early stages of their research careers who are interested in clinical, translational, or basic research on PSC.
- In 2024, the award amounts increased to $100,000 for Young Investigators and $75,000 for Standard Seed Grants (total for 2 years).
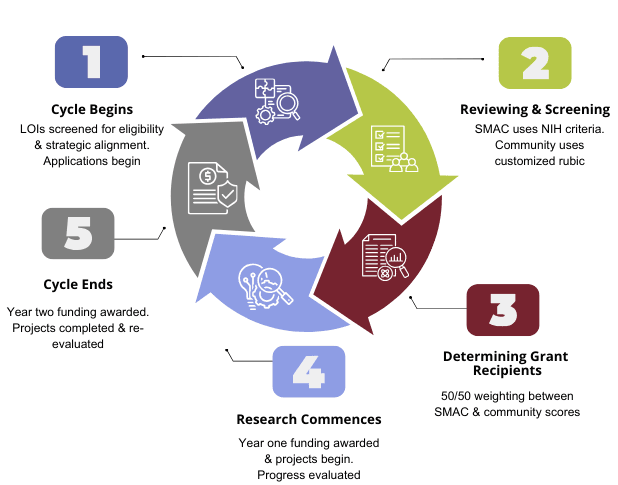
Grants Cycle
The grants program has an annual cycle, starting with letters of intent (LOIs) and progressing to applications, reviews, funding decisions, and reporting:
Grant Reporting Requirements
- PSC Partners staff conduct internal assessments on the grants program's impact, progress, and areas for growth.
- Grant recipients provide a lay summary for the PSC Partners website and a first-year progress report for SMAC to review.
- Second-year funding is contingent upon a successful review of the progress report.
- Grant recipients complete the 2nd year of the research project and submit a final report.
PSC Partners Annual Research Grant Awards
Every year, based on recommendations from the Scientific/Medical Advisory Committee (SMAC) and Community Reviewers, the Board of Directors of PSC Partners reviews all applications and funds the most promising PSC-related studies. In 2024, we announced funding for four international studies, and our affiliate PSC Partners Canada announced funding for one Canadian study.
Grant Application
PSC Partners and affiliate PSC Partners Canada offer grants to conduct research that addresses an important and novel, basic or clinical research question related to PSC and closely associated diseases (such as inflammatory bowel diseases (IBD) and cholangiocarcinoma). Our Research Grants Program seeks to encourage investigators to conduct research in promising new areas, with the goal that data generated will lead to federal (NIH) or external international funding.
Contact us at grants@pscpartners.org.




